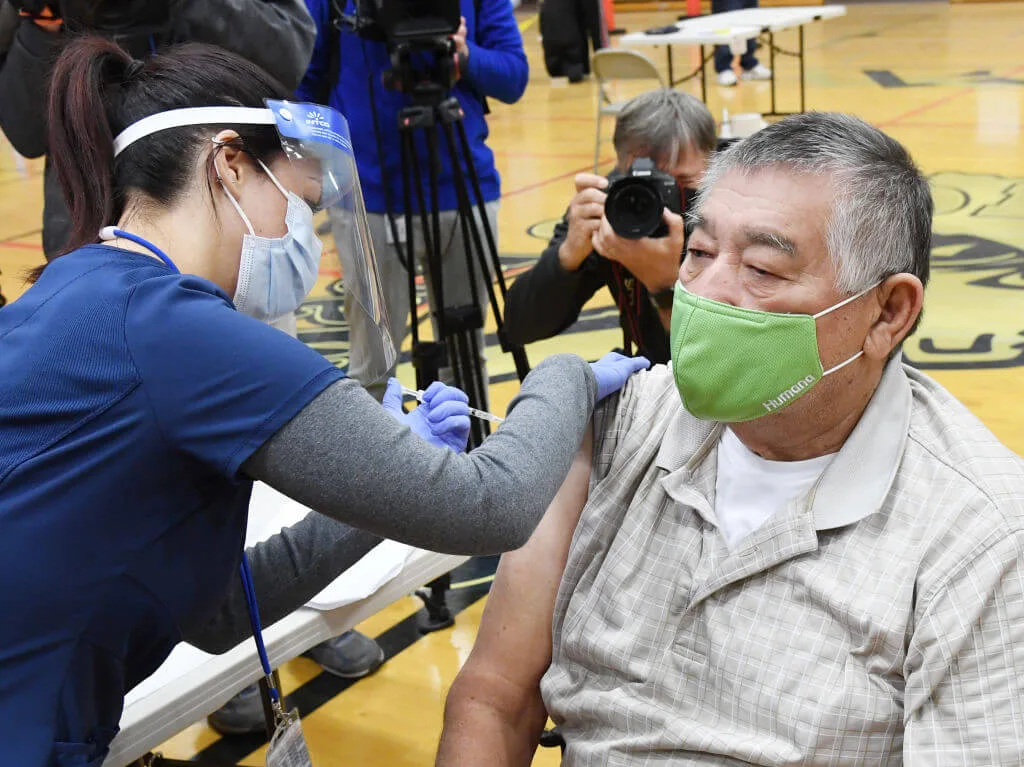
Southern Nevada Health District nurse Daliah Rubio administers a a Pfizer-BioNTech COVID-19 vaccination to Victor Rodriguez, 70, of Nevada, at Jerome Mack Middle School on January 29, 2021 in Las Vegas, Nevada. The Southern Nevada Health District is operating the site as a pop-up clinic for seniors age 70 and older. (Photo by Ethan Miller/Getty Images)
Of the millions of vaccines administered in the United States, only 71 people have had a serious reaction.
More than 22 million Americans have gotten at least one dose of the available COVID-19 vaccines with no serious medical problems beyond allergic reactions, the Centers for Disease Control and Prevention (CDC) reported Wednesday.
Dr. Tom Shimabukuro, deputy director of the CDC’s Immunization Safety Office, presented the agency’s safety data to a CDC advisory committee. The information was collected through several channels, including the CDC’s Vaccine Adverse Event Reporting System, or VAERS, which accepts reports from anyone; the Vaccine Safety Datalink, a collaboration between the CDC and nine major hospital systems that collect data on more than 12 million people annually; V-safe, a self-reporting system for people who’ve been vaccinated; and the Clinical Immunization Safety Assessment Project (CISA), a collaboration between the CDC and vaccine safety experts.
Although side effects are commonly linked to the Pfizer-BioNTech and Moderna vaccines, with 70% of people self-reporting some type of pain, the early tracking systems have found few unusual or dangerous results. Fatigue, headaches, muscle pain, chills, fever, swelling, or joint pain were among the most common effects reported.
Very rarely, anaphylaxis, or severe allergic reactions, occur following vaccination. There have been 71 of these cases reported of the 22 million doses administered; that’s about five instances of anaphylaxis for every million doses of the Pfizer vaccine and three for every million doses of the Moderna vaccine.
Through Jan. 18, 196 people have died after getting a vaccine. Of these deaths, 129 (66%) were patients in long-term care residential facilities. About 1.3 million nursing home residents were vaccinated between Dec. 21 and Jan. 18, and researchers found the number of deaths comparable to expected for that general population. The deaths did not appear to be vaccine-related, according to Dr. Shimabukuro.
“These findings suggest that short-term mortality rates appear unrelated to vaccination for COVID-19,” Shimabukuro said.
More vaccines are on the horizon. The CDC panel also heard an update from AstraZeneca on its developing vaccine, which is currently in Phase III of US clinical trials. The company could be ready to submit for an Emergency Use Authorization by the end of March, with a possible approval by the Federal Drug Administration as early as April.

For Rep. Susan Wild, supporting PA families includes reproductive rights and much more
Rep. Susan Wild wants to be very clear with Pennsylvanians: Donald Trump is committed to taking away women’s reproductive freedom, but he is not...
School districts working with anti-LGBTQ groups can cost your kids’ schools millions
Parents across South Central Pennsylvania are worried about the potential financial impacts working with anti-LGBTQ groups may have on their school...

VIDEO: Trump distances himself from his anti-abortion views
Donald Trump appeared on WGAL on Tuesday and continued to distance himself from his anti-abortion views claiming that reproductive rights are now a...

VIDEO: Community pushback gets school board to rescind decision on denying gay actor’s visit
Cumberland Valley School Board offered a public apology and voted to reinstate Maulik Pancholy as a guest speaker a week after the board voted to...

VIDEO: Project 2025 brings nuclear armageddon back into vogue
Project 2025 is a titanic document, with plans ranging from cutting half of all government employees to targeting reproductive rights on a scale...